From the previous section we observe that for systems for which the potential energy itself does not depend on time, the modulus square of the wave function ![]() given by
given by
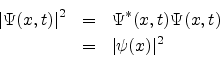
Thus the probability density is independent of time. The state of the system represented by
![]() .
.
is called a stationary state.