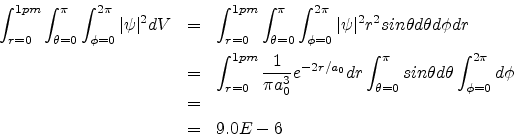Problem 1:
The ground state wavefunction for a particle in a one-dimensional box of length L is
![]()
Suppose the box is 10.0nm long. Calculate the probability that the particle is between x=4.95 nm and 5.05 nm.
Solution:
The probability of finding the particle between x and x + dx is given by
![]() . Therefore the required probability is
. Therefore the required probability is
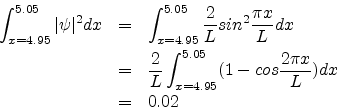
Problem 2:
The ground state wave function of the hydrogen atom is given by
![]()
a0 = 0.53A0 is the Bohr radius. Calculate the probability that the electron would be found somewhere within a small sphere of radius 1pm centred at the nucleus.
Solution:
The required probability is